A Chatteris-based engineering company has announced the appointment of a new director. Advanced Precision Technologies, which specialises in 5-axis CNC programming and manufacturing, has appointed Danny Speirs as a co-director, signalling the start of a phase of planned expansion. Danny brings with him 25 years of manufacturing engineering experience and...
Leveraging Your CE Mark With a Certificate of Free Sale
Are you leveraging your CE mark in order to make your medical devices & IVD devices available in markets outside the European Union (EU)? In many of these markets a Certificate of Free Sale is required to demonstrate EU compliance and forms part of the application process. The medical device...
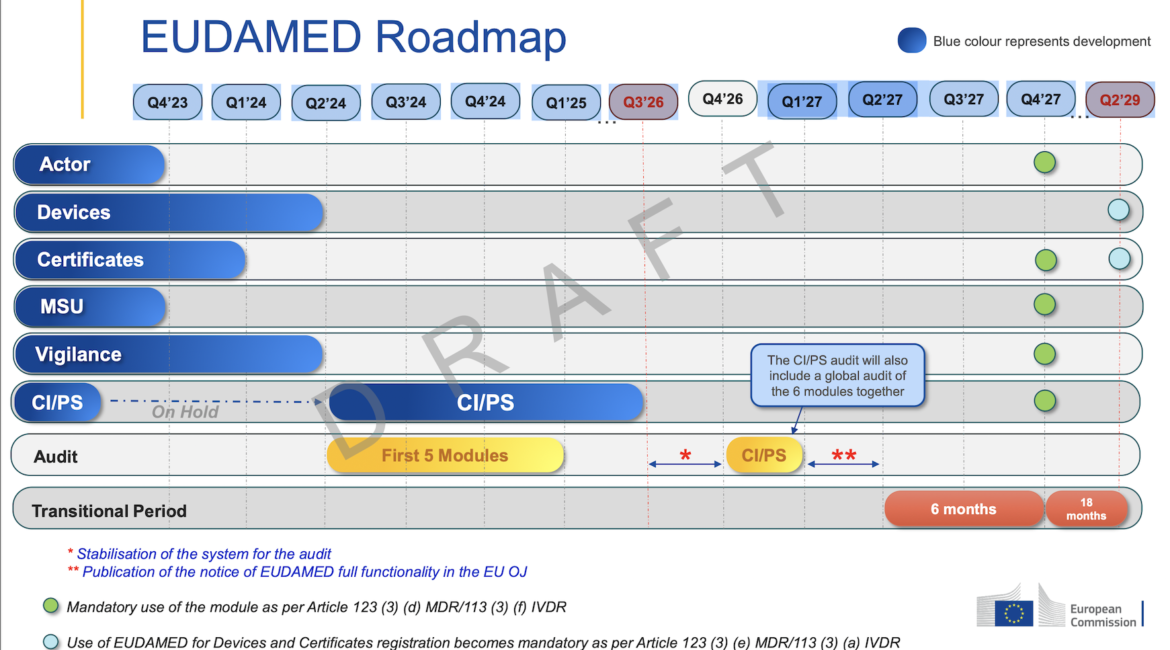
Mandatory Implementation of EUDAMED Delayed Once Again
The EU Commission seemed to have confirmed a delay to the mandatory implementation of EUDAMED by posting a new draft timeline to its website. The draft timeline indicates that all six of the modules will not be in a position to undergo independent audits before Q4 of 2026, this is...
Risk under the microscope: Five challenges facing the life sciences sector
2023 is a pivotal year for UK life sciences as the Government renewed its commitment to increasing funding for research and development, and signed a new deal to re-join the European Union’s €95.5 billion research funding programme known as Horizon Europe. In January, George Freeman, Minister for Science, Research and...
West Midlands duo secures £1.5m Indian export order
Two West Midlands manufacturers are marking 40 years of collaboration by securing an export order at Blechexpo. Brandauer, a leading independent metal stamper, and Auric Metal Finishers from Coventry have partnered to design, develop, and supply customised EloPin push fit-connectors for a tier 1 automotive supplier in India. This £1.5m...
Medilink Midlands celebrates 20 years of supporting businesses
Innovation is the key for growth within the healthcare and Life Sciences sector. Without innovation the sector would stagnate, so it's important that companies and innovators are supported with their great ideas and help them get on their pathway from concept to commercialisation. At Medilink Midlands we are celebrating our...
Boyds launches ‘Conversations in Drug Development’ podcast
Boyds has announced the launch of a brand-new podcast series that aims to keep the scientific and clinical community abreast of the latest advancements in cell and gene therapy and drug development. The podcast – ‘Conversations in Drug Development’ – features candid conversations from the expert team at Boyds, who...
Meet the team: Melanie Davidson, Medilink Midlands CEO
Melanie Davidson is the Chief Executive Officer at Medilink Midlands. We recently caught up with Melanie to find out more about her background, her role and what part, she believes, Medilink Midlands can play in shaping the life sciences and med tech sectors across the Midlands region. How did you...
Meet the Medilink Midlands Chairman, Professor Martin Levermore, MBE DL
Having dedicated his last twenty years to the healthcare sector, Chairman of Medilink Midlands, Professor Martin Levermore, MBE DL, shares his thoughts and experiences of the industry and what benefits the Medilink Midlands can deliver. What is your current role at Medilink Midlands? Currently I’m the chair. I say ‘currently’...
MeTAP Innovation Pre-Accelerator Programme Praised
Created to support pre start-up and established companies in the med tech and life sciences sector located within Nottingham city, the MeTAP business support programme is designed to empower organisations by providing the right knowledge, expertise, skills and connections to help commercialise their innovations. The first of their Pre-Accelerator programmes...