
A major unmet need for a medical device start-up is how to acquire industry benchmarking data or relevant performance data to inform their product development without the substantial time and cost associated with conventional trials. For the fledgling start-up the cost of data acquisition is often perceived as a significant, and sometimes insurmountable hurdle, requiring substantial funding for early stage analysis and subsequent feasibility studies.
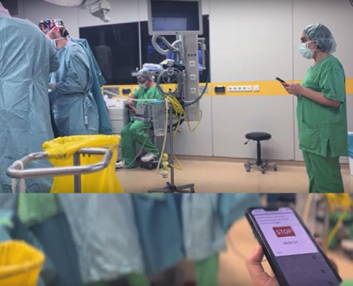
Figure 1 A Surveyor records medical device performance in-use in the operating room. These are qualified at the same level of procedure understanding as a scrub nurse, and work directly with TCC-CASEMIX cloud services. Surgical efficacy, safety and human factors are measured in this process.
TCC-CASEMIX Ltd is now working with start-up manufacturers in general surgery and orthopaedic surgery. The innovation that it has been able to deliver, results in these businesses being able to acquire high quality, multifactorial performance data far faster, far better through greater breadth and depth of data, and at far less cost to their business – all because of TCC-CASEMIX Ltd substantial innovations.
For these businesses the financial impact is either at no net cost to their business, or at a substantially lower cost than conventional methods. For the larger, established manufactures the value proposition works the same way – indeed one of our largest customers informed us recently:
“this is the first time in a clinical study that there has been no net cost to our business – it is a truly innovative market access model that TCC-CASEMIX® delivers.”
TCC-CASEMIX Ltd has achieved this because it has established a TCC-Real World Evidence Centre in Central Europe and through this Centre, where it has deployed its highly innovative TCC-CASEMIX® Technology Platform supported by the world’s first medical device information system, it is able to routinely acquire performance data along the whole surgical pathway, every day of the working week. Further networked Centres are being planned in the UK and in South Korea. It is expected that the first UK one will come on-line in June-July 2023.

Figure 2 A view into the medical device information system providing a substantial resource for medical device manufacturers to understand how their devices perform in-use.
For some manufacturers the business has been able to secure a customer for their product, as part of the data acquisition services. The cost of the service fee from TCC-CASEMIX Ltd then becomes a margin in the cost of the sale. Aside from the substantial cost benefits for manufacturers, the TCC- Real World Evidence Centres offer many significant advantages for the medical device manufacturer, compared to a conventional trials process:
- Ready access to performance data through licensed access to the TCC-CASEMIX® Data Access Portal.
- Real World Evidence data acquisition trials can typically be set up within a 16 week period, including the time required to gain ethical consent.
- No more complexity in trying to find an Investigator to undertake the trial – this comes with the package of services.
- No more complexity in managing the enlisting and screening of patients – this is all taken care of with this unique service.
All of these benefits substantially reduce the time, cost, and complexity of data acquisition. This has to be of substantial value to any medical device manufacturer – not only to the medical device start-up.
These benefits are summed up in this recommendation from a medical device start-up manufacturer:
“TCC-CASEMIX has conceived, planned, and implemented a trial in a very effective timescale. Their attention to detail, meticulous planning and communication has been excellent. As a start-up medical device company, this has been invaluable to my business.
What makes their service so unique is their ability to facilitate a product sale, provide a key opinion leader for the principal investigator role, a fast, efficient, ethical consent process, and patient enrolment in a complete package of services – it is quite exemplary.”
Alberto Casonato, Director, 3D Metal Printing Ltd.
Accelerated Access to foreign markets. TCC-CASEMIX Ltd supports the world’s largest and smallest medical device manufacturers. Currently the business is planning trials in South Korea, and the Indian sub-continent. It is able to deliver its services far and wide, not only because of its innovative cloud- services and its unique Open Registry Infrastructure™ of medical device performance, but also because of the businesses substantial global network.

Figure 3 Open Registry Infrastructure enables licensed users to search for medical device performance wherever the TCC-CASEMIX cloud-services platform has been deployed.
Through its Data Access Portal where medical device performance data is harvested from the medical device information system, the Open Registry Infrastructure™ enables any manufacturer to inspect the performance of its devices anywhere those devices are being used with TCC-CASEMIX®. Now manufacturers have access to a ‘sea of performance data’ for their teams in market access, regulatory compliance, and medical affairs, but without the substantial overhead that characterises much of current practice.
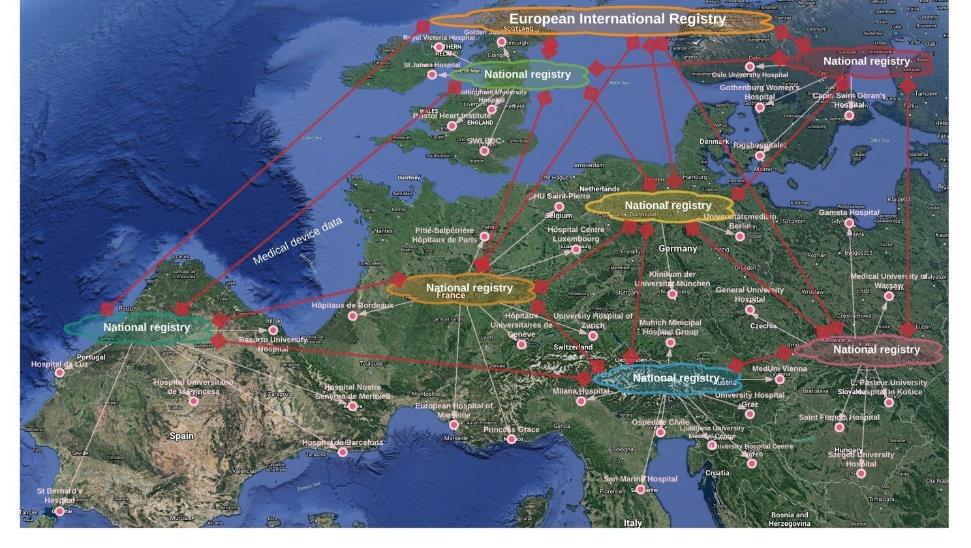
Figure 4 The TCC-CASEMIX Open Registry Infrastructure enables manufacturers to gain access to a veritable ‘Internet of Medical Devices’.
About TCC-CASEMIX Limited. A business that based in the UK, but part of a significant overseas network. It has developed the world’s first cloud-based medical device information system. Aligned to its TCC-CASEMIX® data acquisition platform it is able to acquire real-time data along the whole surgical pathway. It precisely tracks medical devices wherever they are being used at a high level of procedure detail in any operating room in any hospital or indeed in any community setting, where the platform has been deployed.
The business describes its value-proposition as follows:
Multi-award winning TCC-CASEMIX Limited has developed and implemented highly innovative technologies, enabling the routine data acquisition of patient centric datasets aligned with datasets from pre-operative, peri-operative, and post-operative activities for the whole surgical pathway.
The value that this delivers for medical device manufacturers’, surgical service providers and regulators with unique real-world evidence for the multifactorial performance of medical devices used in different surgical specialities, has been described as akin to ‘gold-dust’.
The automated TCC-CASEMIX® data processing centre, deploys algorithms enabling medical devices and associated surgical methods to be directly compared with existing interventions (both therapeutic and surgical) for different subject cohorts. This delivers unique, value-based insights: supporting regulators, and medical device manufacturers to systematically evaluate market differentiation, patient safety, effectiveness of medical device performance on human health, surgical efficacy in the operating room combined with human factors assessments, and finally carbon analytics.
This story was written by Medilink Midlands Member, TCC-CASEMIX Ltd. To find out more about Medilink Midlands membership, click here.